Pharmaceutical Services Programme
Introduction
The Pharmacy Services Programme is one of the programmmes under the Ministry of Health Malaysia (MOH), which is responsible in ensuring that public gets access to safe, efficacious and quality pharmaceutical products, protecting their interest via enforcement of relevant legislations, and ensuring rational use of medicines by both healthcare providers and patients.
Visi, Misi & Objektif
Enhancing our nation’s health through excellence in the practice of pharmacy.
- Uphold legislation and improve policies to ensure the quality of pharmaceutical products and services.
- Ensure effective and responsive organisational system towards sustainable quality services.
- Build capabilities and professionalism through talent development and workforce empowerment
- Intensify collaboration towards best practices and standards.
- To safeguard the nation’s health through scientific excellence in the regulatory control of medicinal products and cosmetics.
The objectives are based on each Divisions in the Pharmaceutical Services Programme, which came into effect on 1st April 2016, through the letter from Human Resource Division, MOH reference Bil. (86) dlm. KK(S) 0.61 BHG. 49 Jld. 2 dated on 8th August 2016. The objectives are in line with the Pharmaceutical Services Programme Strategic Plan 2021-2025.
- National Pharmaceutical Regulatory Agency
- To ensure that therapeutic substances approved for the local market are safe, effective and of quality and also to ensure that notified cosmetic products are safe and of quality.
- Pharmacy Policy & Strategic Planning Division
- Develop policies for the pharmaceutical sector in line with the needs of national health care.
- Ensure excellent and efficient resources management.
- Pharmacy Practice & Development Division
- Ensure continuous access to the quality, safe and cost-effective medicines.
- Strengthen the quality and rational use of medicines towards the better health.
- Enhance pharmaceutical care services in line with the good standards and best practices.
- Pharmacy Enforcement Division
- Ensure all sales, supply, possession and advertising of substances, products and cosmetics are in compliance with the legal provisions.
- Pharmacy Board Malaysia Division
- Ensure the registration of pharmacists and bodies corporate complies with Registration of Pharmacists Act 1951 and its regulations.
- Set the standard for conduct, ethics, proficiency, education and training and Continuous Professional Development (CPD)
Clients' Charter
At Pharmaceutical Services Programme, we strive to provide efficient and effective services to customers based on the following Clients' Charter;
Pharmacy Practice & Development Division
| No | Indicator | Target |
|---|---|---|
|
1. |
Notification to applicant on the result of the listing of medicine(s) into Ministry of Health Medicine Formulary (MOHMF) done within 3 working days from the MOHMF panel meeting date |
100% |
Pharmacy Enforcement Division
| No | Indicator | Target |
|---|---|---|
|
1. |
Issuance of Authorization for the Import & Export of Dangerous Drugs within 7 working days upon receipt of complete application |
100% |
|
2. |
Issuance of Authorization for the Import & Export of Psychotropic Substances within 7 working days upon receipt of complete application. |
100% |
|
3. |
Issuance of e-Permit (Export) for Precursor Chemicals within 7 working days upon receipt of complete application. |
100% |
|
4. |
Issuance of e-Permit (Import) for Controlled Substances within 3 working days upon receipt of complete application. |
100% |
|
5. |
Decision for application of medicines advertisement certificate and healthcare services advertisement certificate (other than website advertisement) made within 5 working days upon receipt of complete application. |
90% |
|
6. |
Decision for application of medicines advertisement certificate and healthcare services advertisement certificate for website advertisements made within 30 days upon receipt of complete application. |
90% |
|
7. |
Complaint related to infringement of Pharmacy Laws responded within 14 working days. |
100% |
|
8. |
Complaint sample completed analysis by Pharmacy Enforcement Division Chemical Forensic Laboratory within 30 working days from the sample receipt date. |
90% |
|
9. |
Digital device analysis report for investigative purposes will be issued by the Cyber Forensics Laboratory within 120 days from the date the complete application is received |
75% |
Pharmacy Board Malaysia Division
| No | Indicator | Target |
|---|---|---|
|
1. |
Issuance of Full Registration of Pharmacist’s Certificate within 7 working days upon receipt of complete application and in accordance with legislation. |
100% |
|
2. |
Issuance of Registration of Body Corporate’s Certificate within 6 working days upon receipt of complete application and in accordance with legislation. |
100% |
|
3. |
Issuance of Annual Certificate of Pharmacist within 6 working days upon receipt of complete application and in accordance with legislation. |
100% |
|
4. |
Issuance of Registration of Provisional Pharmacist’s Certificate within 7 working days upon receipt of complete application and in accordance with legislation. |
100% |
|
5. |
Issuance of Annual Certificate of Body Corporate within 6 working days upon receipt of complete application and in accordance with legislation. |
100% |
National Pharmaceutical Regulatory Agency
| No | Indicator | Target |
|---|---|---|
|
1. |
FULL EVALUATION: |
100% |
|
2. |
FULL EVALUATION: |
100% |
|
3. |
FULL EVALUATION: |
100% |
|
4. |
FULL EVALUATION: |
100% |
|
5. |
FULL EVALUATION: |
100% |
|
6(a). |
ABRIDGED EVALUATION: |
100% |
| 6(b). | ABRIDGED EVALUATION: Evaluation of Product Registration Application for Generics (Non-Scheduled Poison) with two (2) or more active ingredients (Timeline: 136* working days) *upon receipt of complete application |
100% |
|
7(a). |
ABRIDGED EVALUATION: |
100% |
| 7(b). | ABRIDGED EVALUATION: Evaluation of Product Registration Application for Health Supplements (General and Functional Claims) with two (2) or more active ingredients (Timeline: 120* working days) *upon receipt of complete application |
100% |
|
8(a). |
ABRIDGED EVALUATION: |
100% |
| 8(b). | ABRIDGED EVALUATION: Evaluation of Product Registration Application for Natural Products (Traditional and Homeopathic) with two (2) or more active ingredients (Timeline: 120* working days) *upon receipt of complete application |
100% |
| 8(c). | ABRIDGED EVALUATION: Evaluation of Product Registration Application for Natural Products (Natural Products with Modern Claim) with single active ingredient (Timeline: 116* working days) *upon receipt of complete application |
100% |
| 8(d). | ABRIDGED EVALUATION: Evaluation of Product Registration Application for Natural Products (Natural Products with Modern Claim) with two (2) or more active ingredients (Timeline: 136* working days) *upon receipt of complete application |
100% |
|
9. |
Issuance of Cosmetic Notification (Timeline: 1* working day) * For applications that meets the specified requirements |
100% |
| 10. |
Approval of Change of Registration Holder Application *upon receipt of complete application |
100% |
| 11. | Approval of Change of Manufacturing Site (Timeline: 60* working days) * upon receipt of complete application |
100% |
| 12. | Approval for Manufacturer’s, Import, and Wholesaler’s License (Timeline: 4* working days) * upon receipt of complete application |
100% |
| 13(a). | Evaluation of Clinical Trial Import Licence (CTIL) and Clinical Trial Exemption (CTX) application involving First-in-Human trials, biologics, Cell & Gene Therapy Products (CGTPs) and Herbal products (Timeline: 45* working days) *upon acceptance of complete application |
100% |
| 13(b). | Evaluation of Clinical Trial Import Licence (CTIL) and Clinical Trial Exemption (CTX) application involving investigational products other than stated above (13(a)). (Timeline: 30* working days) *upon acceptance of complete application |
100% |
| 14(a). | Issuance of Certificate Of Free Sale (CFS) for Cosmetic (Timeline: 15* working days) *upon receipt of complete application |
100% |
| 14(b). |
Issuance of Certificate Of Free Sale (CFS) for Veterinary Product *upon receipt of complete application |
100% |
| 15. |
Issuance of Certificate of Pharmaceutical Product (CPP) *upon receipt of complete application |
100% |
| 16. | INSPECTION ACTIVITIES: GMP/GDP/GCP/GLP/BE/EC inspection reports must be issued within 30 working days after the inspection. [Note: GMP (Good Manufacturing Practice), GDP (Good Distribution Practice), GCP (Good Clinical Practice), GLP (Good Laboratory Practice), BE (Bioequivalence), EC (Ethics Committee)] |
100% |
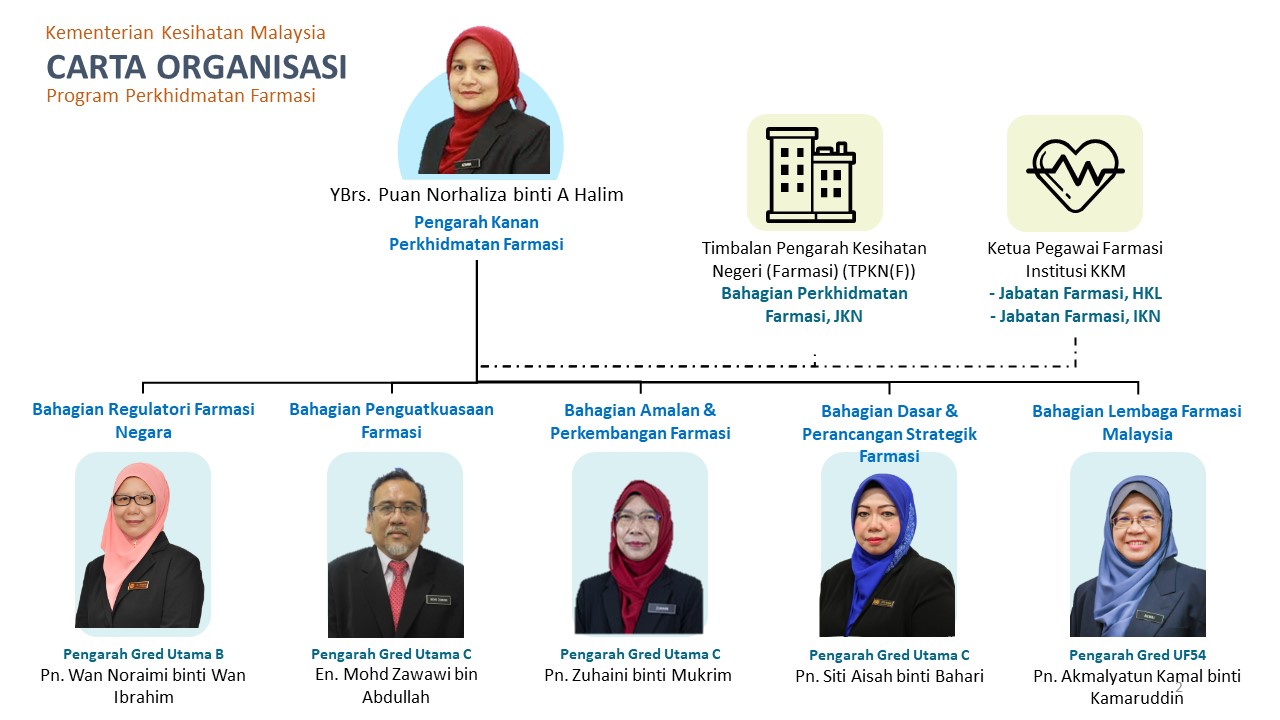
Carta Organisasi
Soalan Lazim
Pemohon boleh menghubungi pihak sekretariat Lembaga Iklan Ubat melalui panggilan telefon (03-7841 3200), emel (
Hubungi Kami
Alamat: Lot 36, Jalan Universiti (Jalan Universiti, 46200 Petaling Jaya, Selangor
Tel : 03 - 78413200
Faks : 03 - 79682222
E-mel :
Laman Web : pharmacy.gov.moh.my